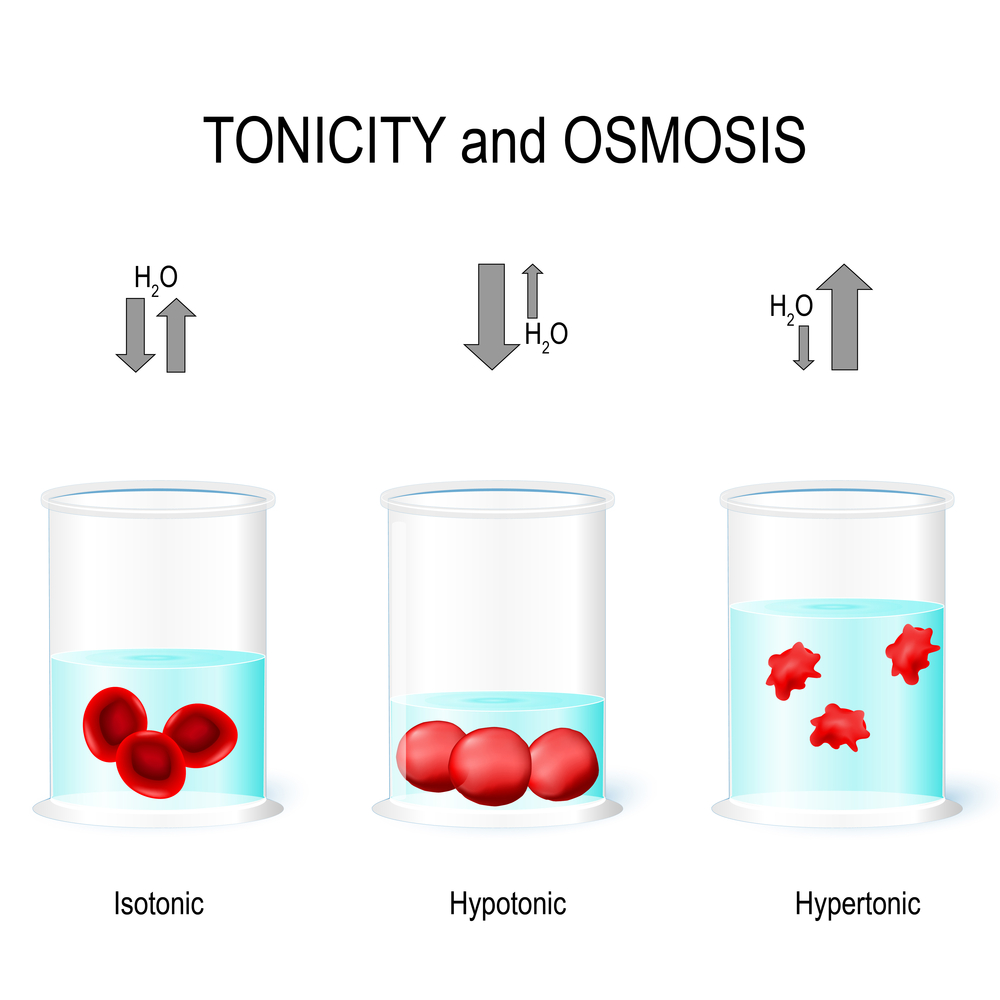
An isotonic solution is one that has the same osmolarity, or solute concentration, as another solution. If these two solutions are separated by a semipermeable membrane, there will be no net movement of water across the membrane. This balance is crucial in various biological and medical contexts, ensuring that cells maintain their shape and function without experiencing excessive swelling or shrinking.
The Science Behind Isotonicity
Osmolarity refers to the total concentration of solute particles in a solution. In a biological context, cells typically exist in an isotonic environment to maintain homeostasis. For example, human red blood cells thrive in an isotonic solution, where the osmotic pressure is equal inside and outside the cell. This equilibrium prevents water from entering or leaving the cell excessively, thus avoiding conditions like hemolysis (bursting) or crenation (shrinking).
Applications in Medicine
Isotonic solutions are commonly used in medical settings, particularly in intravenous (IV) therapy. Solutions such as 0.9% sodium chloride (normal saline) and lactated Ringer’s solution are considered isotonic with human blood plasma. These solutions are administered to patients to restore or maintain fluid balance, especially in cases of dehydration or blood loss.
The use of isotonic solutions is not limited to IV therapy. They are also crucial in various laboratory procedures, such as cell culture, where maintaining the osmotic balance is vital for cell survival and growth. Isotonic conditions help researchers study cellular responses and interactions without the confounding effects of osmotic pressure changes.
Why Isotonicity Matters
Maintaining isotonic conditions is essential for several reasons:
- Cell Integrity: Isotonic solutions help preserve cell structure and function, which is critical for overall health.
- Effective Treatments: Using isotonic solutions in medical treatments minimizes complications and ensures that therapeutic agents are delivered effectively.
- Research Reliability: In laboratory settings, isotonic solutions provide a controlled environment for studying biological processes, leading to more accurate and reproducible results.
Conclusion
Understanding isotonic solutions is fundamental in both biological and medical fields. Their role in maintaining osmotic balance is crucial for the proper functioning of cells and for the success of various treatments. Whether in a clinical setting or a research laboratory, isotonicity plays a pivotal role in promoting health and understanding life’s complex processes. As science continues to evolve, the significance of isotonic solutions will undoubtedly remain a cornerstone of both medical practice and biological research.